Abstract
Introduction
The composition of the intestinal microbiota is associated with important outcomes after allo-HSCT including survival, relapse, GVHD, and infections. The microbial composition changes drastically after allo-HSCT, with precipitous drops in α-diversity and domination by single organisms. Low diversity is particularly associated with poor overall survival from transplant-related mortality, including death after GVHD. These observations were all made in single-center cohorts. Here we compare-for the first time-the kinetics of microbiota diversity in allo-HSCT patients from three independent international institutions. We also explore the role of nutrition as well as antibiotics in a single-center pilot study and the recovery from microbiota injury after discharge.
Methods
We collected stool samples approximately weekly from adult allo-HSCT inpatients and approximately monthly post-discharge. Samples from all three centers were sequenced at MSKCC using the V4-V5 region of 16S rRNA. In the nutrition cohort, patients self-documented oral intake at each meal; data were confirmed in focused patient interviews by a registered dietician three times weekly and nutritional compositions were obtained from the hospital kitchen database. Oral swish samples were collected prior to allo-HSCT and during post-transplant neutropenia.
Results
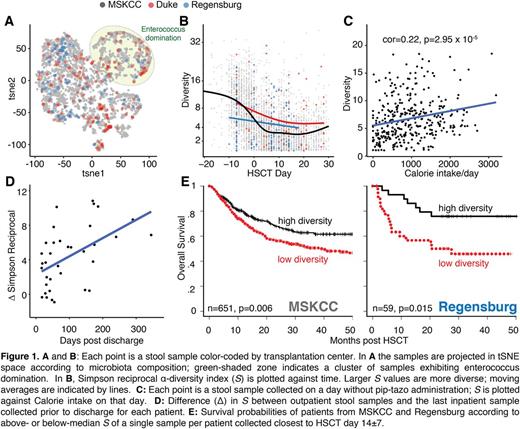
5,823 samples from 1,118 patients were compared: 5,508 samples from 947 MSKCC patients, 108 samples from 40 Duke patients, and 152 samples from 109 Regensburg, Germany patients. The patients-all adult recipients of allo-HSCT -varied in underlying diagnosis, donor-graft sources, conditioning intensity, and GVHD prophylaxis. We mapped the microbiota composition of each sample in 2-D space using t-Distributed Stochastic Neighbor Embedding (t-SNE). Samples from all three centers spread throughout t-SNE space, revealing no transplant-center-specific effect (Fig 1A). Enterococcus domination, a low-diversity state we previously associated with subsequent enterococcal bacteremia was observed in all three centers (Fig 1A). Diversity decreased comparably after allo-HSCT transplant at all centers (Fig 1B), validating our prior findings in this larger, international multicenter cohort. In a separate cohort of MSKCC patients, we explored the relative contributions of antibiotics and nutritional intake using a multivariate linear regression model of α-diversity dynamics. Exposure to piperacillin-tazobactam (pip-tazo), a drug with broad-spectrum antibacterial activity, and calorie intake were significant predictors (p<0.01 and p<0.05, respectively). Samples collected on days of pip-tazo administration had lower diversity as compared to pip-tazo-free days (Simpson reciprocal diversity index (S): 4.15±0.35 vs 7.75±0.21, p<10-175). Calorie intake correlated positively with diversity in the absence of pip-tazo (r=0.22, p=2.95x10-5; Fig 1C). The oral microbiota in 12 of these patients also showed a post-transplant decrease in α-diversity (S pre-HSCT 4.90 ± 0.79 and post-HSCT 7.76±0.97, p=0.034); this drop was associated with exposure to broad-spectrum antibiotics (p = 0.016). Recovery from microbiota injury after allo-HSCT-investigated in 37 samples from a separate cohort of 21 outpatients -showed remarkable gradual recovery of microbiota diversity at a rate in S of 2.17 per 100 days (Fig. 1D, p=0.004). Finally, we validate our prior association of low intestinal α-diversity around the time of neutrophil engraftment with poor overall survival in a larger cohort from MSKCC (n = 651, p = 0.006 for below- vs. above-median S) and in a cohort from Regensburg with evaluable samples from this timepoint (n = 59, p = 0.015) (Fig 1E).
Conclusion
We determine that striking microbiota injuries after allo-HSCT occur generally across geographic regions. Nutritional perturbations and antibiotic exposure are both associated with microbiota injury, and recovery from the injured state occurs over several months post-discharge. Importantly, diversity at the time of neutrophil engraftment is associated reproducibly with overall survival.
Peled: Seres: Research Funding. Sung: Merck: Research Funding; Novartis: Research Funding; Cellective: Research Funding. Jenq: Seres: Research Funding. van den Brink: Jazz Pharmaceuticals: Consultancy; Seres: Research Funding; PureTech Health: Consultancy; Therakos Institute: Other: Speaking engagement.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract